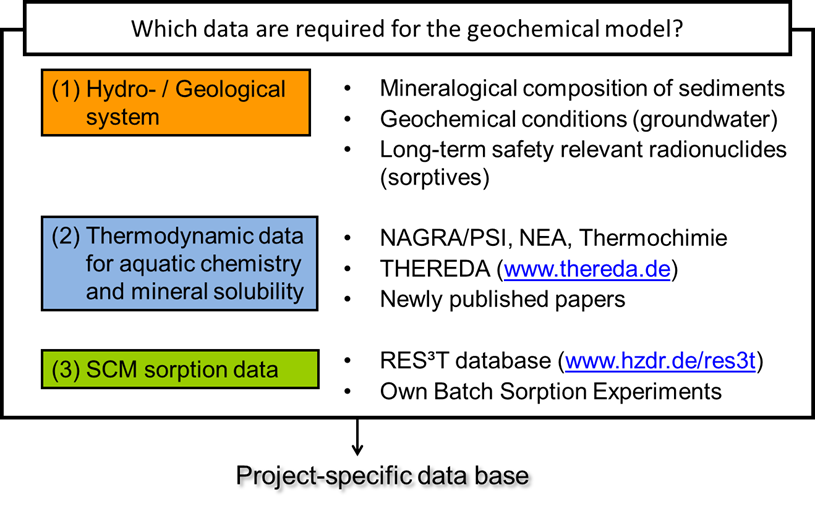
Thermodynamic Data
Fig. 1 provides an overview about the thermodynamic data required for the modelling of transport and sorption processes. The selection of the data (relevant elements and mineral phases) of course depends on the application cases as described in “Application ”.
Fig. 1 Scheme of thermodynamic data required for parameterization of smart Kd-models.
As the salinity of different groundwater types calls for different models for activity coefficients for aqueous species, two separate thermodynamic databases (TDB) were created: • For solutions with ionic strengths lower than 0.5 mol L-1 the Davies approach (Davies, 1962) based on the Extended Debye-Hückel Theory (EDH) models ion-ion interactions. • For highly saline solutions the Pitzer formalism (Pitzer, 1991) is applied to model ion-ion interactions. It follows a short description of (1) data for the aquatic chemistry containing reaction constants for dissolved species and solid mineral phases, and (2) sorption data for surface complex formation constants. The elements, species, and solids covered by these databases are defined by the requirements for the specific application cases.
Aquatic Chemistry
TDB for low mineralized water: The basis for the EDH based TDB is the PSI/Nagra TDB 12/07 formatted for PHREEQC (Thoenen et al., 2014). In addition, data for Fe(II)/(III) were updated from the NEA TDB Vol. 13a (Lemire et al., 2013) and for U(VI) from THEREDA Update Uranium Revision 2.1.1. Moreover, solubility products for albite, anorthite, chlorite, illite, and montmorillonite were taken from the ANDRA Database ThermoChimie-TDB (Version PHREEQC_eDH_v9b0.dat, Giffaut et al., 2014). The dissolution of K-feldspar was approximated by the solubility product for microcline as published by Stefánsson and Arnórsson (2000). The solubility of muscovite and an amorphous Gibbsite phase were taken from Richter et al. (2016) and Lindsay (1979), respectively. TDB for highly mineralized water: The Pitzer TDB is based on the THEREDA database (www.thereda.de, Moog et al., 2015). This provides quality assured thermodynamic data for the radionuclides and fission products (Am(III), Nd(III), U(IV/VI), Np(V), Th(IV), Tc(IV & VII), Sr(II) and Cs(I)) as well as for matrix elements (the hexary system of oceanic salts including carbonates and phosphates), and concrete materials (cementitious phases with Al and Si).
Sorption data
The thermodynamic data for surface species for relevant sorbates (pair of element and mineral) were critical evaluated and selected from the sorption database RES³T (Rossendorf Expert System for Surface and Sorption Thermodynamics) (Brendler et al., 2003; www.hzdr.de/res3t). This database is mineral-specific and thus applicable for additive models of more complex solid phases such as rocks, sediments, or soils. Various Surface Complexation Models (SCM) are included therein. So far, the project database exclusively utilizes the 2pK Diffuse Double Layer Model (DDLM) described by Dzombak and Morel (1990) as it is rather simple and robust, currently has the most extensive parameterization for complex systems, and it is supported by most geochemical speciation programs. The set-up of a sorption database followed this strategy:
- A literature survey mainly based on the RES³T database provided a list of all available 2pK DDLM data records for the system components of relevance.
- Values for specific surface area were partly substituted by experimentally determined ones.
- To keep the system as simple as possible, no distinction is made between strong and weak binding sites.
- All surface complexation protolysis constants are converted to infinite dilution by assigning activity coefficients based on the Davies-Equation (Davies, 1962) to all dissolved species.
- Because the reported reaction constants are related to different site densities, they were converted following Kulik (2002) to a reference surface site density of 2.31 sites nm2.
- After normalization, all records for the same reaction (mineral surface protolysis and surface complexation) are compared and assessed to identify and exclude outliers and doubtful data points. The remaining sets are then averaged to obtain respective surface complexation parameters and also an estimation of their uncertainty.
A few critical data gaps concerning sorption data were closed by own experimental efforts as described in Lab.
Contact: Dr. Madlen Stockmann
References:
Brendler, V., Vahle, A., Arnold, T., Bernhard, G., Fanghänel, T.: RES3T - Rossendorf expert system for surface and sorption thermodynamics. J. Cont. Hydrol., 61, (2003) 281–291.
Davies, C. W.: Ion Association. Butterworths, Washington (1962). Dzombak, D.A., Morel, F.M.M.: Surface complexation modeling. Hydrous ferric oxide, John Wiley & Sons, New York (1990). Giffaut, E., Grivé, M., Blanc, P., Viellard, P., Colàs, E., Gailhanou, H., Gaboreau, S., Marty, N., Madé, B., Duro, L.:
ANDRA thermodynamic database for performance assessment: ThermoChimie. Appl. Geochem. 49, (2014) 225-236. Kulik, D.A.: Sorption modelling by Gibbs energy minimisation: Towards a uniform thermodynamic data base for surface complexes of radionuclides. Radiochim. Acta, 90, (2002) 815-832.
Lemire, R., Berner, U., Musikas, C., Palmer, D.A., Taylor, P., Tochiyama, O.: Vol. 13a. Chemical Thermodynamics of Iron Part I. OECD Nuclear Energy Agency Data Bank, Eds., OECD Publications, Issy-les-Moulineaux (2013).
Lindsay, W.L.: Chemical equilibria in soils. John Wiley & Sons, New York (1979).
Moog, H. C., Bok, F., Marquardt, C., Brendler, V.: Disposal of Nuclear Waste in Host Rock formations featuring high-saline solutions: Implementation of a Thermodynamic Reference Database (THEREDA). Applied Geochemistry 55, (2015) 72–84.
Pitzer, K.S.: Activity coefficients in electrolyte solutions (2nd ed.). Chapter 3: Pitzer, K.S. Ion interaction approach: theory and data correlation, pg. 75–153, C.R.C. Press. (1991).
Richter, C., Müller, K., Drobot, B., Steudtner, R., Großmann, K., Stockmann, M., Brendler, V.: Macroscopic and spectroscopic characterization of uranium(VI) sorption onto orthoclase and muscovite and the influence of competing Ca2+. Geochim. Cosmochim. Acta 189, (2016) 143–157.
Stefánsson, A., Arnórsson, S.: Feldspar saturation state in natural waters. Geochim. Cosmochim. Acta 64, (2000) 2567–2584.
Thoenen, T., Hummel, W., Berner, U., Curti, E.: The PSI/Nagra Chemical Thermodynamic Database 12/07, PSI Report 14-04 (available for download at http://www.psi.ch/les/database) (2014).