A central aspect within the SMILE project is the characterization of sorption complexes on the molecular level. This is an essential basis for the correct chemical description of sorption reactions, and thus the extraction of thermodynamic parameters. The partners specifically characterize sorption processes in those systems where data gaps are identified.
We use modern spectroscopic, microscopic, and diffraction techniques to characterize sorption complexes and the reactions leading to their formation. Initial studies focus on mica, feldspar, and hematite and their interaction with low-valent actinides, e.g. Am(III), Pu(III), and Th(IV). Additional research will then be driven by findings in these studies as well as input from titration and column experiments, and thermodynamic modelling and SCM.
Time-resolved laser-induced fluorescence spectroscopy (TRLFS)
Further characterization of sorption structures of U(IV, VI), as well as Cm(III) and Am(III), can be realized using time-resolved laser-induced fluorescence spectroscopy (TRLFS). The fluorescence spectra enable distinction between different possible surface processes (incorporation, inner sphere sorption, outer sphere sorption) and a species distribution can be obtained. It is possible to draw conclusions about the hydration sphere of actinide ions with lifetime measurements.
See here for more details about the experimental technique.
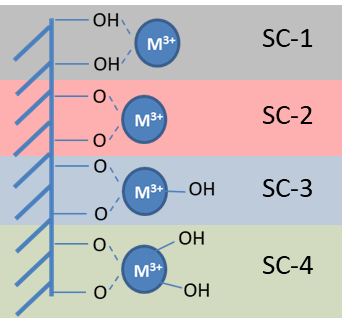
Fig.: 1 Cm(III) sorption species on K-feldspar, obtained by TRLFS.
For example, TRLFS was used to determine the sorption structure of Cm(III) on K-feldspar(Neumann et al., 2020) depending on pH and Cm concentration. Four different surface complexes (SC-1 to 4), a complex in which the Cm3+ is attached to the neutral mineral surface, an inner sphere complex and two hydrolysis forms, were identified (Fig. 1).
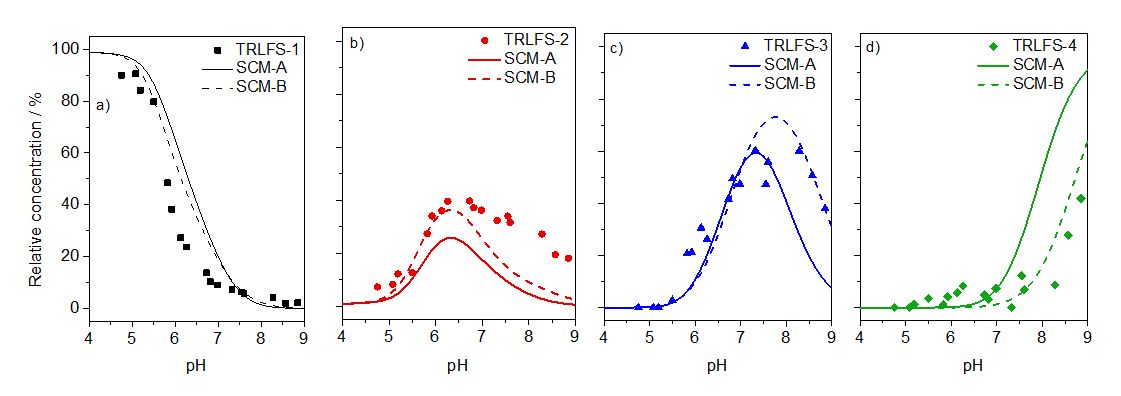
Through peak deconvolution, the quantitative contributions of each species was determined. The results are exemplarily displayed in Fig. 2.
Fig. 2: Species distribution derived from TRLFS (symbols) and fit of M3+ speciation under conditions of the 5 µM Cm TRLFS series, and simulated speciation using SCM-A (without TRLFS, solid lines) and SCM-B (with TRLFS, dashed lines). TRLFS species 1 represents the sum of the M3+ aquo ion and SC-1, TRLFS species 2 represents SC-2, aq. Cm-silicate and (alumo)silicate colloids, TRLFS species 3 represents SC-3, TRLFS species 4 represents SC-4 (cf. Table 2, Table 3, and Table S4 (SI)).
The structural information was used together with batch sorption data to develop a reliable and robust surface complexation model. See details (Neumann et al., 2020).
Surface X-ray Diffraction (CTR/RAXR)
A major role will be played by CTR (crystal truncation rod diffraction) and RAXR (resonant anomalous X-ray reflectivity), modern surface-specific diffraction methods that use high-energetic, brilliant X-ray beams at synchrotrons. These methods enable in situ determination of crystal- and sorption structures, processes at the water-mineral interface and real-time measurements of reactions at the mineral surface with sub-Å resolution (Fenter et al. (2002), Park et al. (2007)).
Measurements are currently implemented at the Advanced Photon Source (Argonne National Laboratory) in the United States. Additionally HZDR is operating its own beamline (ROBL) at the European Synchrotron Radiation Facility (ESRF) in Grenoble (France).
Currently, the influence on background electrolyte composition on the interfacial formation of Th(IV) nanoparticles on mica (001) is studied using this technique in combination with in situ AFM. See here for more details (Schmidt et al. (2012), Schmidt et al. (2015)).
Contact: PD Dr. Moritz Schmidt
References:
Neumann, J., Brinkmann, H., Britz, S., Lützenkirchen, J., Bok, F., Stockmann, M., Brendler, V., Stumpf, T., Schmidt, M., 2020: A comprehensive study of the sorption mechanism and thermodynamics of f-element sorption onto K-feldspar, J. Colloid Interface Sci. 2020. https://doi.org/10.1016/j.jcis.2020.11.041.
Fenter, P., X-ray Reflectivity as a Probe of Mineral-Fluid Interfaces: A User Guide. Rev. Min. Geochem. 2002, 49, 149-220.
Park, C.; Fenter, P., Phasing of Resonant Anomalous X-ray Reflectivity Spectra and Direct Fourier Synthesis of Element-Specific Partial Structures at Buried Interfaces. J. Appl. Crystallogr. 2007, 40 (2), 290-301.
Schmidt, M.; Lee, S. S.; Wilson, R. E.; Soderholm, L.; Fenter, P. Sorption of Tetravalent Thorium on Muscovite. Geochim. Cosmochim. Acta 2012, 88, 66–76. https://doi.org/10.1016/j.gca.2012.04.001.
Schmidt, M.; Hellebrandt, S.; Knope, K. E.; Soo, S.; Stubbs, J. E.; Eng, P. J.; Soderholm, L.; Fenter, P. Effects of the Background Electrolyte on Th ( IV ) Sorption to Muscovite Mica. Geochim. Cosmochim. Acta 2015, 165, 280–293. https://doi.org/10.1016/j.gca.2015.05.039.