The surface charge of mineral surfaces is typically determined by electrokinetic or potentiometric methods. For sufficiently small particles (< 1 µm) electrokinetic mobility measurements and acid-base titrations can be carried to out to determine the point(s) of zero charge and the pH-dependent zeta-potential as well as the proton related surface charge density (Delgado et al. (2007), Lützenkirchen et al. (2012)).
Some particles may be so large in size that either they settle in an electrokinetic mobility measurement or that they do not exhibit sufficiently large specific surface area to generate so much total surface area in a batch experiment that reliable acid-base titrations can be done. In such cases the zeta-potential can be obtained from streaming potential measurements (Lützenkirchen et al., 2010). Acid-base type data can be collected via column titrations (Scheidegger et al. (1994), Neumann et al. (2020)).
Ideally, the experiments are not only carried out as a function of pH but also for various background electrolyte concentrations. These data can be used to construct surface protolysis models for a given mineral. The models are required for a comprehensive surface complexation model to describe solute (contaminant) adsorption to the mineral.
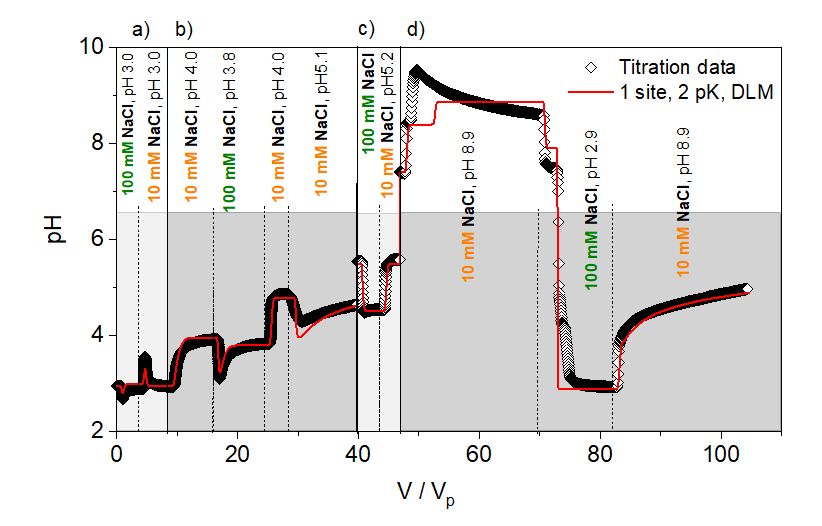
Figure 1: Experimental pH (symbols) as a function of V/Vp (V: eluted volume, Vp: pore volume) and fitted results (solid red line) of K-feldspar column titrations using a 1 site, 2 pK DLM model: pKa = 2.5 ± 0.02, CEV = 0.007. Dashed vertical lines illustrate change of titrant, solid vertical lines represent a start of a new experiment, a) – d) above the figure respectively. Each experiment started with an equilibrated column using the first electrolyte.
Contact: Dr. Johannes Lützenkirchen, Dr. Susan Britz
References:
Delgado, A.V., et al., Measurement and interpretation of electrokinetic phenomena. Journal of Colloid and Interface Science, 2007. 309(2): p. 194-224.
Lützenkirchen, J., et al., Potentiometric titrations as a tool for surface charge determination. Croatica chemica acta, 2012. 85(4): p. 391-417.
Lützenkirchen, J., et al., An attempt to explain bimodal behaviour of the sapphire c-plane electrolyte interface. Advances in Colloid and Interface Science, 2010. 157(1): p. 61-74.
Scheidegger, A., et al., Convective transport of acids and bases in porous media. Water Resources Research, 1994. 30(11): p. 2937-2944.
Neumann, J., Brinkmann, H., Britz, S., Lützenkirchen, J., Bok, F., Stockmann, M., Brendler, V., Stumpf, T., Schmidt, M., 2020: A comprehensive study of the sorption mechanism and thermodynamics of f-element sorption onto K-feldspar, J. Colloid Interface Sci. 2020. https://doi.org/10.1016/j.jcis.2020.11.041.